Trialbee is a global technology provider for patient matching and retention in clinical trials. Retaining patients in clinical trials is a challenge since lengthy trial processes with slow-moving signs of condition improvement often lead to frustrated patients, causing non-adherence and early drop-outs. Trialbee offers software solutions that apply key design principles to maximise patient engagement and adherence.
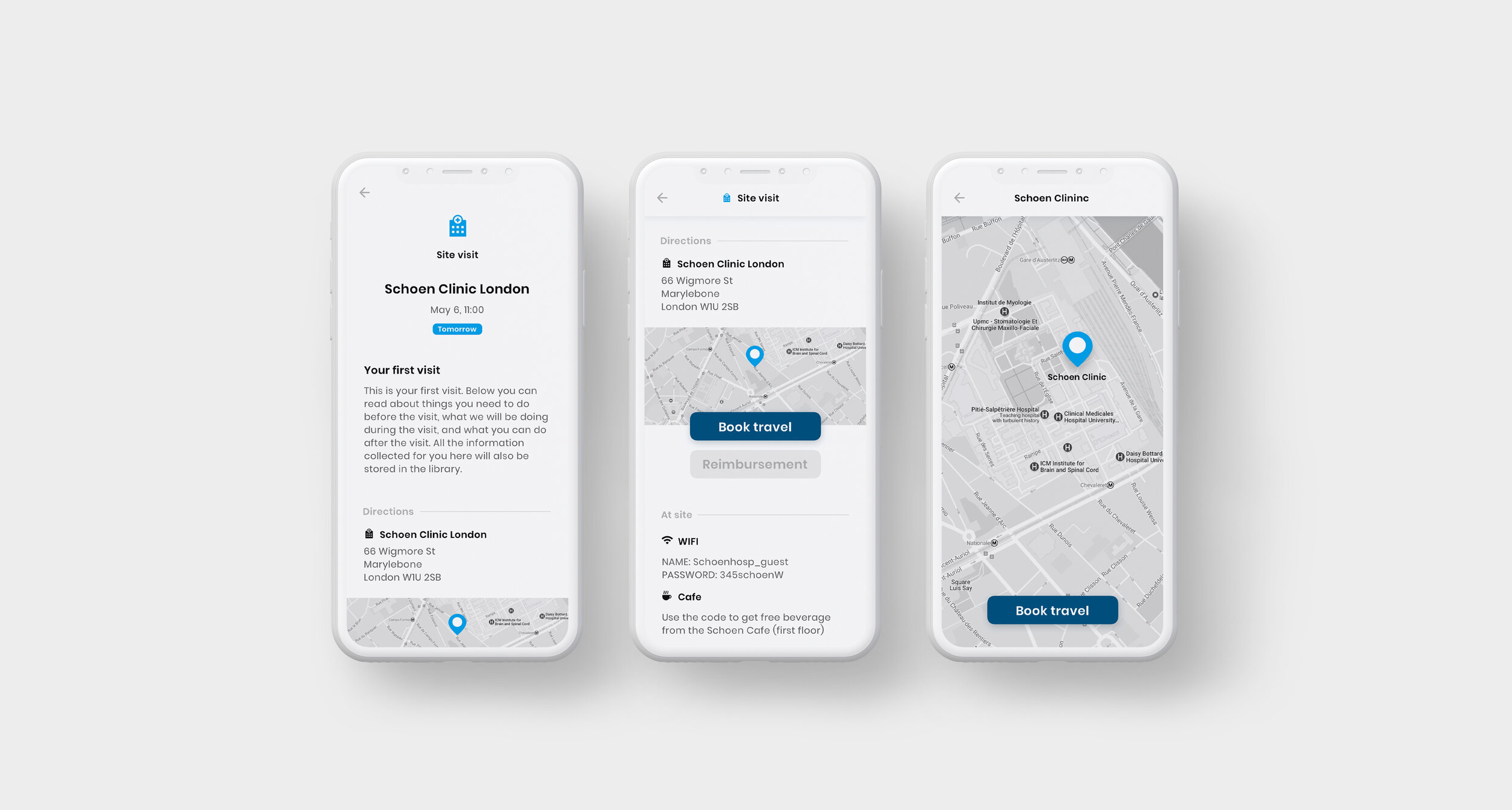
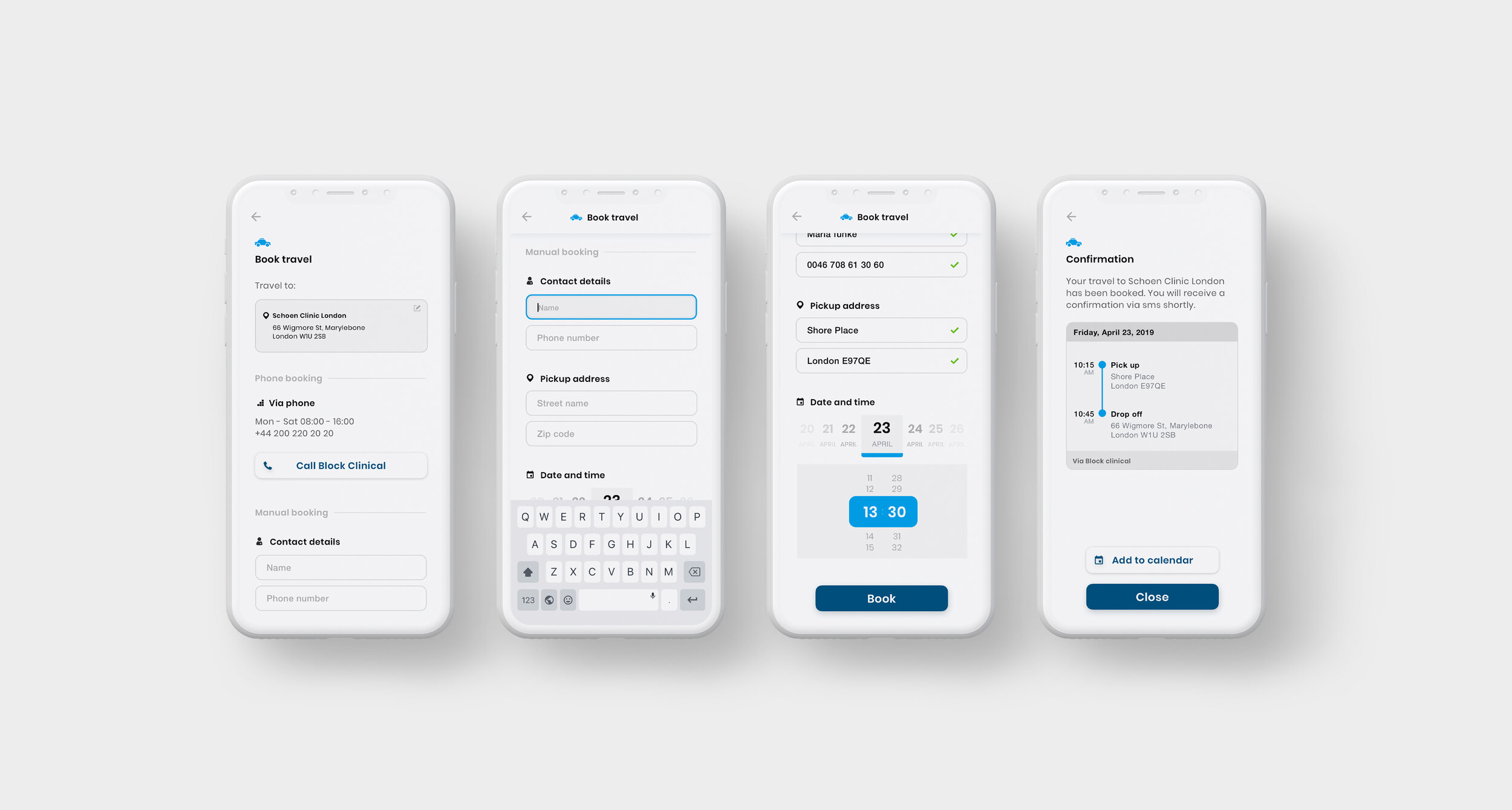
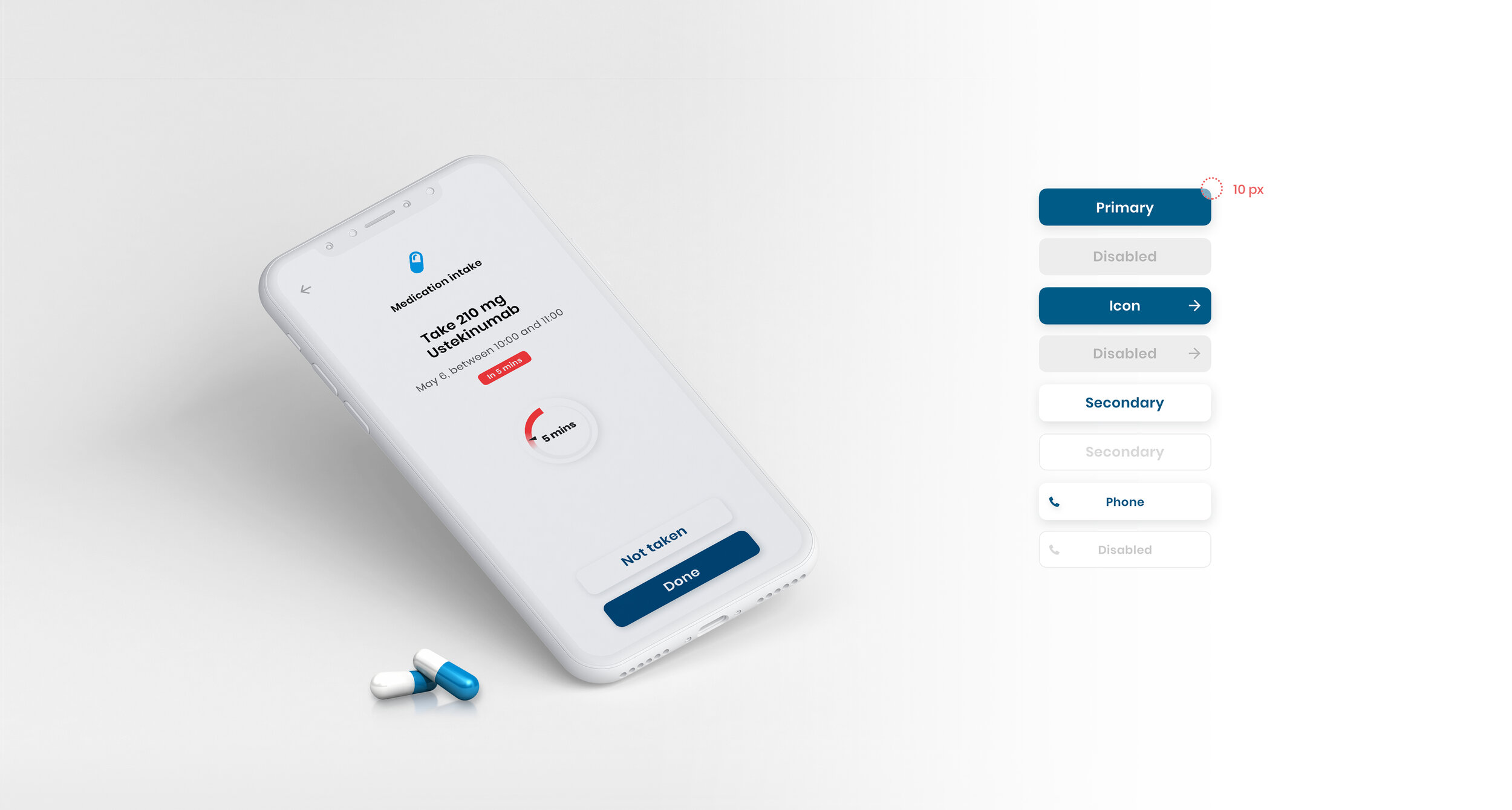
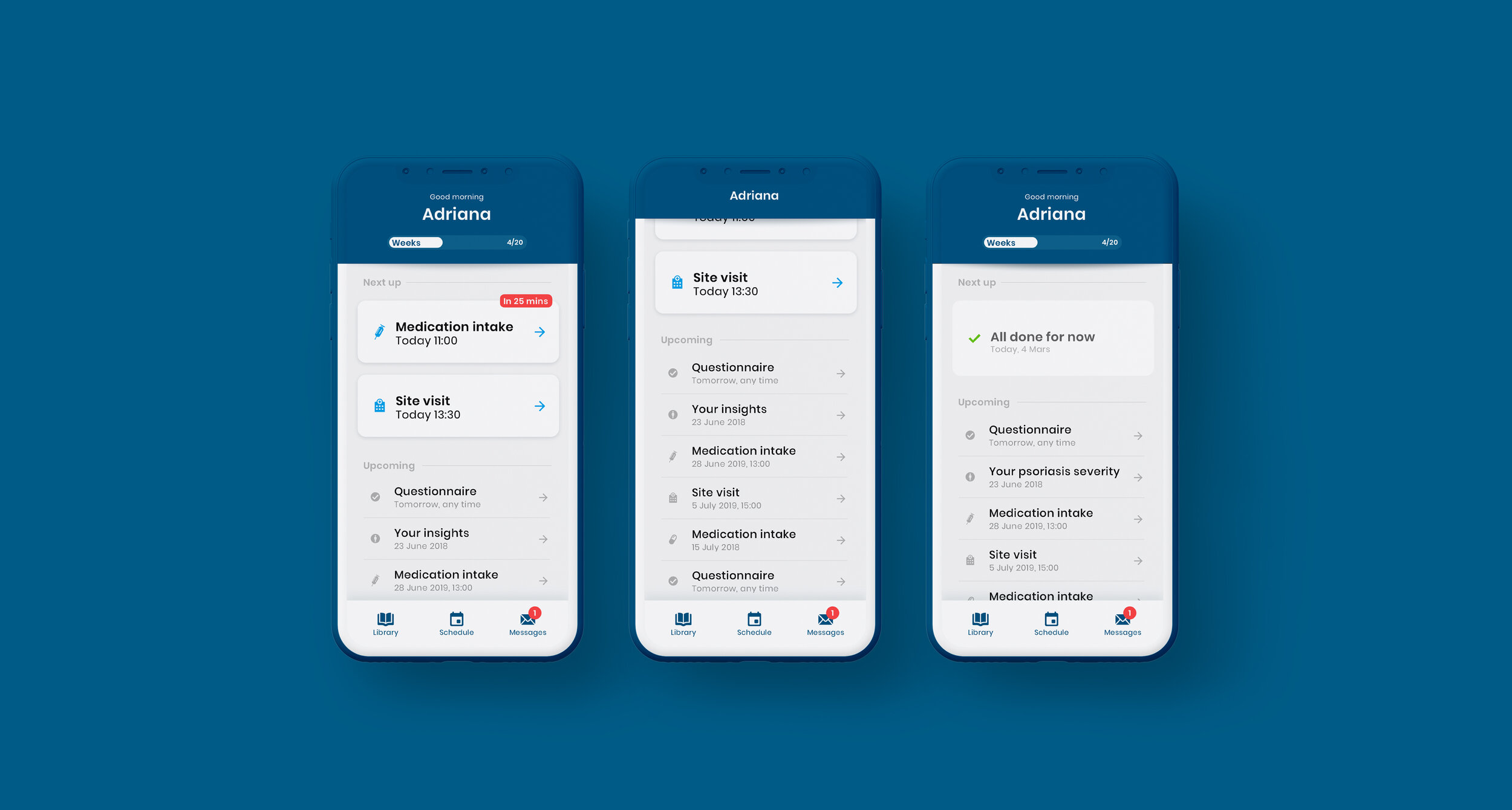
As part of the Patient Engagement team at Trialbee, I worked together with trial specialists and developers to evaluate, adjust and re-design existing applications including a mobile app (showcased below), developed to empower patients with tools and information related to a clinical trial. The goal of the app re-design was to improve the UI to better meet the needs of our stakeholders, caregivers and patients so as to meet them where they are digitally, physically and emotionally. In this project, the UI was designed based on the Trialbee brand for sales purposes, proof of concept, prototyping and testing. The design was created and implemented in the form of a UI library – refined and improved iteratively together with the development team.
Deliverables: UX, UI re-design, component library, prototype
In collaboration with team at Trialbee. Not to forget the great designers before me who initiated the project and paved the way.
Year: 2018
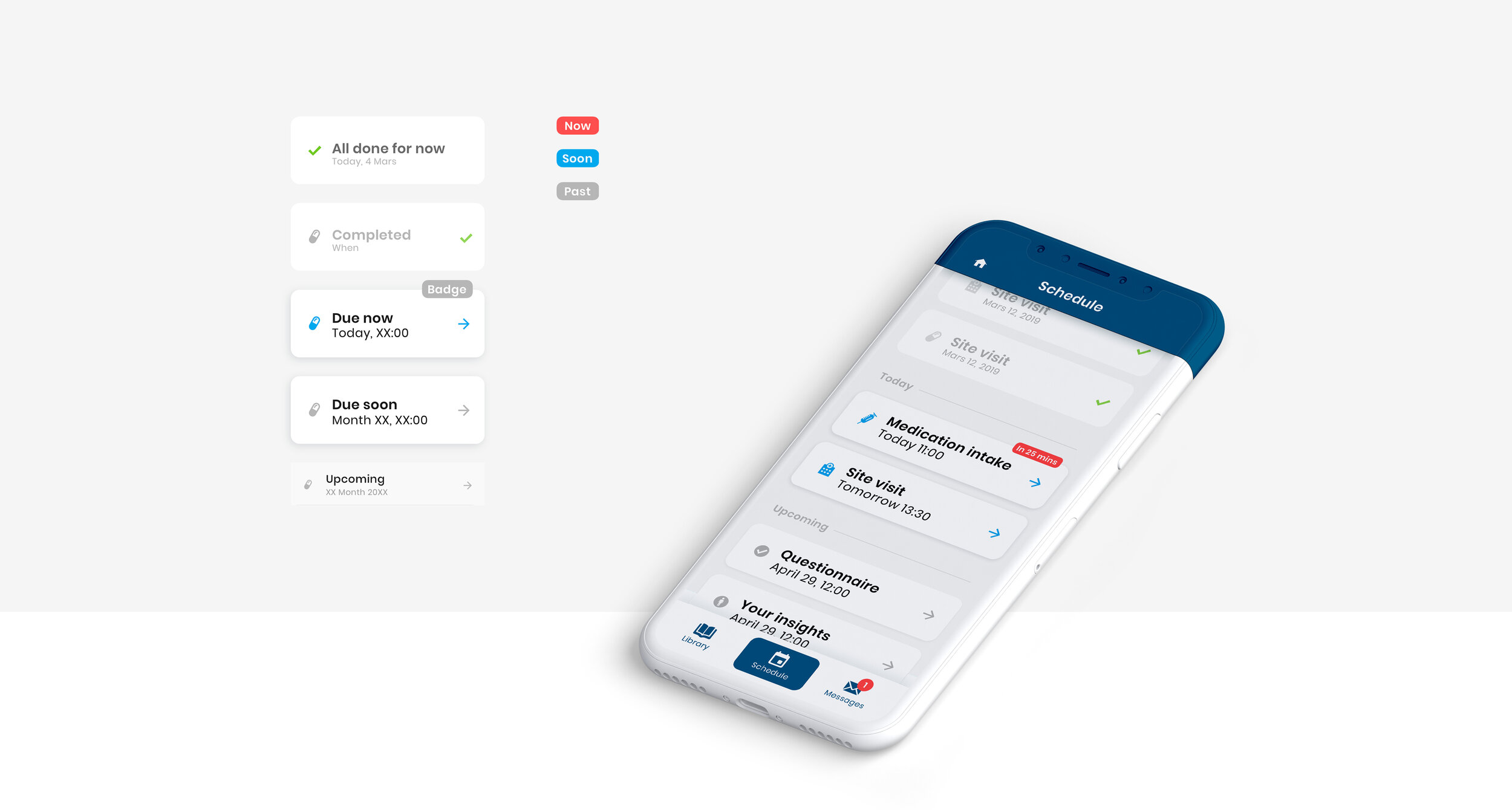


As part of re-design, we collected statistical data by analysing cases and trends in clinical trial drop-outs. In addition, we gathered qualitative information involving relevant stakeholders such as pharma clients, caregivers and patients to gain deeper understanding of the problem from a clinical and behavioural perspective. On my part, I conducted on-to-one interviews with patients whereby findings were combined with statistical data and delivered in the form of an empathy map/patient journey. This material served as a visual representation of the emotional experience that patients go through during trial participation and was used as guidance for re-defining the product requirements and relevant features before re-design.